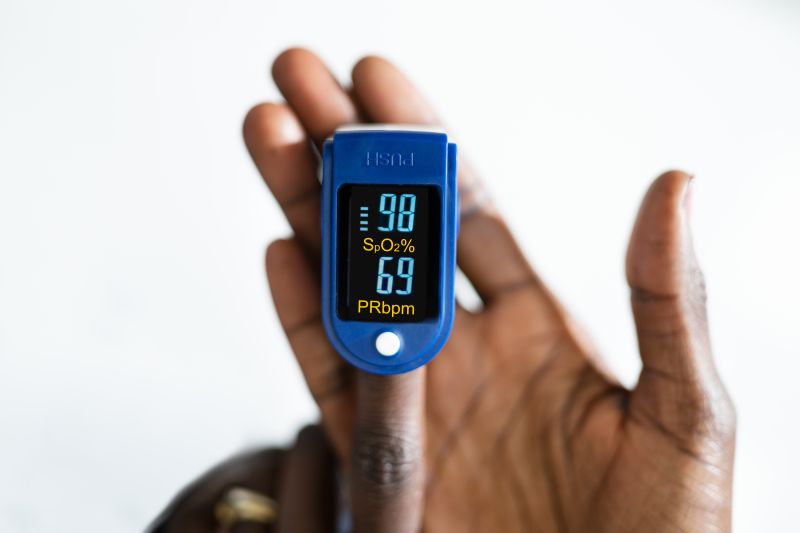
Years of research have showed that pulse oximeters yield less accurate readings for people with darker skin tones, and now the US Food and Drug Administration is proposing guidance to help make these devices more reliable and less biased.
Pulse oximeters are small finger-clamp devices that estimate how much oxygen is being carried in the blood. Available both over the counter and by prescription, they grew in popularity during the Covid-19 pandemic. But many studies have revealed that pulse oximeters can measure blood oxygen levels as higher than they actually are for people with dark skin.
The FDA announced Monday that it has published draft guidance for manufacturers of pulse oximeters that offers recommendations for the clinical testing and labeling of these electronic medical devices.
One of the recommendations is to include “a diversely pigmented group of 150 or more healthy participants” in clinical studies of the devices, with at least 25% of participants falling within each skin color group on the system known as the Monk Skin Tone scale.
Another is for manufacturers to “prominently display appropriate warnings” in the devices’ instructions, such as informing patients that “differences in skin pigmentation may cause differences in pulse oximeter sensor performance.”
“This draft guidance is aligned with the FDA’s broader commitment to helping facilitate the development of high-quality, safe, and effective medical devices,” Dr. Michelle Tarver, director of the FDA’s Center for Devices and Radiological Health, said in the agency’s announcement. “Our draft recommendations are based on the best available science to help address concerns of disparate performance of pulse oximeters based on an individual’s skin pigmentation.”
The FDA is requesting public comments on the draft guidance within 60 days, and then the agency will review and consider the comments before finalizing the guidance.
How pulse oximeters work
Pulse oximeters clamp onto a fingertip and send light beams through the finger to estimate pulse rate as well oxygen levels of the blood. A sensor on the other side of the device receives those light beams and uses them to detect the color of a person’s blood. Bright red blood is highly oxygenated, while blue or purplish blood is less so.
If these devices are not calibrated for darker skin tones, the pigmentation of the skin could affect how that light is absorbed by the sensor, leading to erroneous oxygen readings.
Pulse oximeters were invented in 1974, and the evidence of flawed pulse oximeter readings in people with dark skin dates to the 1980s.
One study, published in 2022, found that among more than 3,000 hospitalized patients receiving intensive care, those who were Asian, Black and Hispanic received less supplemental oxygen than White patients, which was associated with differences in their pulse oximeter readings.
Another paper published in 2022 found that Black patients had higher odds than White patients of having low blood oxygen measured in their blood-drawn readings but not detected by pulse oximetry – so a higher likelihood of inaccuracies.
A separate study of about 7,000 Covid-19 patients, also published in 2022, found that compared with White patients, blood oxygen levels measured using pulse oximetry were overestimated by an average of 1.7% among Asian patients, 1.2% among Black patients and 1.1% among Hispanic patients. That overestimation may have contributed to a patient not receiving certain Covid-19 therapies when they needed the care.
The FDA held two advisory panel meetings — in 2022 and 2024 — to discuss the accuracy of pulse oximeters and ways to improve and evaluate their performance, and the agency issued a safety communication warning that the devices have limitations and may be less accurate in people with dark skin pigmentation.
The FDA noted that the new draft guidance applies to pulse oximeters that are intended for medical purposes, which are primarily used in hospital settings and doctor’s offices, and not for devices sold as general wellness products, which are often sold directly to consumers over-the-counter.
Some pulse oximeters that are on the market may already meet the updated recommendations, according to the FDA, but if so, that should be indicated on their labeling.
The agency also proposed creating a public webpage identifying all of the pulse oximeters that have been FDA-cleared for medical purposes and have undergone FDA review under the new draft guidance.
‘We can only hope’
The recommendations are not binding, and a new federal administration is taking office shortly, said Carmel Shachar, faculty director of the Health Law and Policy Clinic at Harvard Law School, who was not involved in the draft guidance.
“It is challenging timing to put it out now and then have an administration switch over and have people be taking it back up while figuring out what are going to be the priorities of this new administration. So there’s a concern there,” Shachar said.
There are also concerns that manufacturers may not fully comply with the FDA guidance, said Dr. Theodore J. Iwashyna, professor of pulmonary and critical care medicine and of health policy and management at Johns Hopkins University.
In 2013, the FDA issued premarket guidance for developers of pulse oximeters in which it recommended that they have “a range of skin pigmentation” represented in their clinical testing of the devices, including at least two “darkly pigmented subjects or 15% of the study group, whichever is larger.” But a research paper published last week in the journal JAMA found low uptake of that suggestion.
“Given how little compliance was seen when the last voluntary guidance was released a decade ago, we can only hope that the FDA finalizes and enforces these,” Iwashyna, who was not involved in the FDA update, said in an email Monday. “Unless there is formalization, enforcement, and compliance, I am not sure why we would anticipate these ‘proposed new draft recommendations’ would result in better products, and therefore better, more equitable care.”
Some public health experts also worry that many over-the-counter pulse oximeters may not be included in the recommendations. But overall, Shachar said, she wants to see the new draft guidance fully implemented.
“I do really like the framing of it, in that it’s trying to provide a lot of carrots for manufacturers to do the right thing, to engage in the studies to make sure that there isn’t bias in their devices,” Shachar said.
Sign up here to get The Results Are In with Dr. Sanjay Gupta every Friday from the CNN Health team.
“If you do that, and you do that with a meaningful number of subjects – 150 – then this guidance says you can get a label saying that your device performs comparably across patient groups and potentially get on a registry or some sort of public-facing list that the FDA is going to compile,” she said. “It’s trying to perhaps walk a fine line. It’s not trying to do a recall of biased devices, that’s for certain. It’s ‘we’re going to incentivize these good products to come on market in order to label them in a way to encourage consumers to use them.’ ”
Iwashyna added that the new guidance should provide more detail as to how standards are set for what the FDA considers sufficiently non-disparate performance in patients with darker skin when it comes to both performance and safety.
“The current document also proposes very general tolerances for ‘equitable’, that seem to be based on what current devices can do, rather than what is actually safe for patients with darker skin,” he said. “I think our patients deserve proven safety, not made-up safety thresholds.”